Our Clinical Programs
FRIEDREICH ATAXIA CARDIOMYOPATHY (FA-CM)
Friedreich ataxia (FA) is a multisystemic genetic disease — involving the nervous system and heart — in which heart issues are the leading cause of a shortened lifespan. Though considered rare, FA is the most common form of hereditary ataxia in the U.S.

FA is caused by a change, or mutation, of the frataxin (FXN) gene. This change in the FXN gene means that less frataxin protein is made (remember, the incorrect recipe!). Without frataxin, the body cannot make enough energy to power organs and tissues like the brain, muscles and heart.

Most people can recognize the signs and symptoms associated with FA, such as difficulty with movement, muscle weakness, poor muscle control and unsteady balance.1 But what often goes unnoticed is the silent damage to the heart.

Heart issues associated with FA are particularly dangerous because they can begin silently but can eventually lead to complications such as heart failure and abnormal heart rhythms.2,3

There currently are no approved treatments for heart disease in FA, which makes it crucial to perform clinical trial research for treating the heart-related issues in FA.4
At Lexeo, we have made this unmet need a top priority and are currently researching an investigational gene therapy called LX2006 in clinical trials.
ARRHYTHMOGENIC CARDIOMYOPATHY (ACM)
Arrhythmogenic cardiomyopathy (ACM), also known as arrhythmogenic right ventricular cardiomyopathy (AVRC), is a heart disease characterized by loss and replacement of myocardial tissue with fibrofatty tissue and scarring. This process disrupts electrical conduction and increases arrhythmia risk and disruptions in normal heart rhythm. If left unmanaged, ACM can lead to structural changes in the heart, which increase the risk of heart failure. Sudden cardiac death (SCD) becomes a notable concern in advanced stages.4
In the United States, studies show that 23% of individuals with ACM present with SCD as their first symptom, highlighting its severity.4

Mutations in Desmosomal Genes Can Lead to ACM
Desmosomal genes make proteins that help cells connect to one another to help stabilize the structure of tissues, like the heart. Mutations in these genes can compromise the structural stability of the heart and disrupt the electrical system that keeps the heart functioning correctly.4
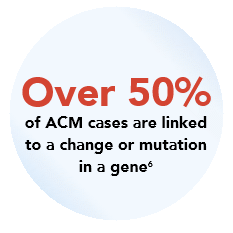
ACM is most commonly caused by a change or mutation in one of the desmosomal genes called plakophilin-2 (PKP2).4 We refer to ACM caused by changes to the PKP2 gene as PKP2-ACM.
The PKP2 gene codes for the PKP2 protein, which is critical for desmosome function in heart cells. PKP2 gene mutations reduce PKP2 protein levels, leading to structural and mechanical instability in heart muscle cells, resulting in PKP2-ACM. Over time, this instability contributes to damage to the heart cells, and the heart muscle starts to get replaced by fibrous or fatty tissue.4

Over time, damage to the heart due to PKP2-ACM worsens, increasing the risk of SCD and severe heart complications. Current treatments, such as implantable defibrillators and medications, focus on symptom management and SCD prevention but do not address the underlying cause of heart dysfunction, nor halt disease progression.4,7
This Highlights a Critical Need for More Targeted Therapeutic Approaches.
At Lexeo, we have made this unmet need a top priority through our research of an investigational gene therapy called LX2020.
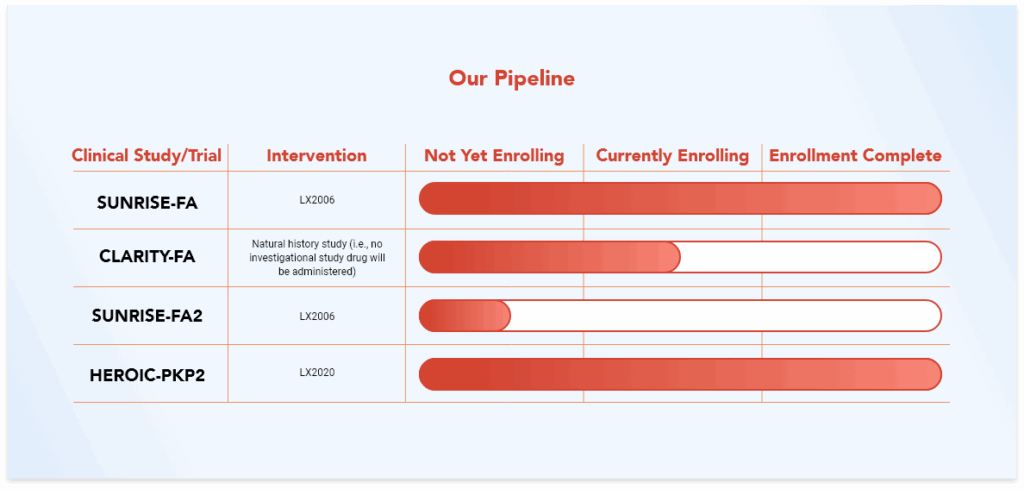
Learn More About Our Trials and Studies

HEROIC-PKP2 is a clinical trial evaluating the safety and health effects of LX2020 in people with PKP2-ACM.

The CLARITY-FA natural history study is designed for researchers to learn about how heart disease develops and worsens in individuals with FA.
References
- Payne RM. Cardiovascular Research in Friedreich Ataxia. JACC: Basic to Translational Science. 2022;7(12):1267-1283. doi:10.1016/j.jacbts.2022.04.005
- Norrish G, Rance T, Montanes E, et al. Friedreich’s ataxia-associated childhood hypertrophic cardiomyopathy: a national cohort study. Arch Dis Child. 2022;107(5):450-455. doi:10.1136/archdischild-2021-322455
- Hanson E, Sheldon M, Pacheco B, Alkubeysi M, Raizada V. Heart disease in Friedreich’s ataxia. World J Cardiol. 2019;11(1):1-12. doi:10.4330/wjc.v11.i1.1
- Data on file.
- van Opbergen CJM, Noorman M, Pfenniger A, et al. Plakophilin-2 Haploinsufficiency Causes Calcium Handling Deficits and Modulates the
Cardiac Response Towards Stress. International Journal of Molecular Sciences. 2019;20(17):4076. doi:10.3390/ijms20174076 - Lexeo Therapeutics. Data on file.
- Calore M, Lorenzon A, De Bortoli M, Poloni G, Rampazzo A. Arrhythmogenic cardiomyopathy: a disease of intercalated discs. Cell Tissue Res.
2015;360(3):491-500. doi:10.1007/s00441-014-2015-5 - van der Voorn SM, te Riele ASJM, Basso C, Calkins H, Remme CA, van Veen TAB. Arrhythmogenic cardiomyopathy: pathogenesis, pro-arrhythmic
remodelling, and novel approaches for risk stratification and therapy. Cardiovasc Res. 2020;116(9):1571-1584. doi:10.1093/cvr/cvaa084
