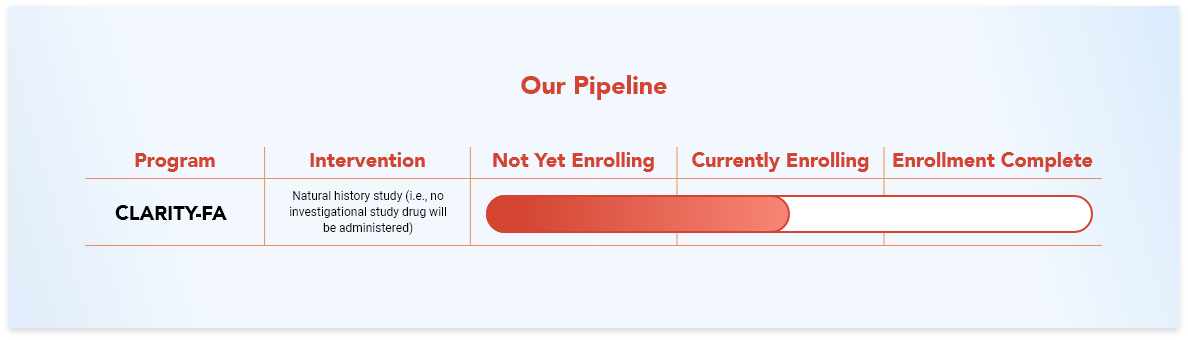
The goal of the Lexeo sponsored CLARITY-FA natural history study is to learn about how heart disease develops and worsens in individuals with Friedreich ataxia (FA). This is important because even though approximately 80% of people with FA develop heart muscle disease, researchers currently do not know much about how the heart is impacted in FA.1
The information from CLARITY-FA study will also help advance our gene therapy research.

What Is a Natural History Study and Why Is It Important?
In order to develop treatments for a disease, researchers must first learn more about it. A natural history study is a type of research that tracks how a disease develops and changes over time in individuals who have it.
Researchers observe symptoms, changes and patterns to better learn what to expect from the disease, find ways to diagnose it earlier and how to manage the disease better.

Information from natural history studies provides important knowledge that can help develop better or new treatments for a disease.
It is important to note that participants in natural history studies do not receive any investigational study drug as the goal is to learn about the natural course of the disease.
Overview*
Condition
The study is open to individuals with genetically confirmed FA diagnosis and evidence of cardiomyopathy based on specific criteria.
Population
The study includes two age groups: Individuals who are at least 16 years of age and individuals who are between 6 and 16 years of age.
Duration
If you participate in this study, you will be asked to visit the study site 3 to 4 times over a 52-week period.
*Full eligibility criteria will be evaluated by the clinical study or trial doctor to determine if an individual is the right candidate for this study.
Important Information
- This study is sponsored by Lexeo Therapeutics, Inc.
- Participation in the CLARITY-FA study is voluntary.
- You may withdraw from the study at any time for any reason. If you refuse to participate or decide to withdraw, you will not suffer any penalty, loss of rights, or loss of benefits to which you are entitled.
- You will receive no direct payment for taking part in the study.
- Participants and their caregiver will be reimbursed for reasonable study-related expenses for travel (e.g., transportation, meals, and hotel, as appropriate).
- As with any clinical study or trial, there may be risks involved with participation. We encourage any individual considering participation in a clinical study or trial to consult with your physician or medical team.
Locations
Multiple sites across the U.S.; sites will be listed shortly.
Sign up below for more information.
References
1. Payne RM. Cardiovascular Research in Friedreich Ataxia. JACC: Basic to Translational Science. 2022;7(12):1267-1283. doi:10.1016/j.jacbts.2022.04.005
